Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022
Weekly / January 28, 2022 / 71(4);109–117
Please note:. This report has been corrected.
Miwako Kobayashi, MD1; Jennifer L. Farrar, MPH1; Ryan Gierke, MPH1; Amadea Britton, MD1,2; Lana Childs, MPH3; Andrew J. Leidner, PhD1; Doug Campos-Outcalt, MD4; Rebecca L. Morgan, PhD5; Sarah S. Long, MD6; H. Keipp Talbot, MD7; Katherine A. Poehling, MD8; Tamara Pilishvili, PhD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Currently, the 13-valent pneumococcal conjugate vaccine (PCV) (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23) are recommended for U.S. adults. Recommendations vary by age and risk groups.
What is added by this report?
On October 20, 2021, the Advisory Committee on Immunization Practices recommended 15-valent PCV (PCV15) or 20-valent PCV (PCV20) for PCV–naïve adults who are either aged ≥65 years or aged 19–64 years with certain underlying conditions. When PCV15 is used, it should be followed by a dose of PPSV23, typically ≥1 year later.
What are the implications for public health practice?
Pneumococcal vaccination recommendations were simplified across age and risk group. Eligible adults may receive either PCV15 in series with PPSV23 or PCV20 alone.
In 2021, 20-valent pneumococcal conjugate vaccine (PCV) (PCV20) (Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.) and 15-valent PCV (PCV15) (Merck Sharp & Dohme Corp.) were licensed by the Food and Drug Administration for adults aged ≥18 years, based on studies that compared antibody responses to PCV20 and PCV15 with those to 13-valent PCV (PCV13) (Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.). Antibody responses to two additional serotypes included in PCV15 were compared to corresponding responses after PCV13 vaccination, and antibody responses to seven additional serotypes included in PCV20 were compared with those to the 23-valent pneumococcal polysaccharide vaccine (PPSV23) (Merck Sharp & Dohme Corp.). On October 20, 2021, the Advisory Committee on Immunization Practices (ACIP) recommended use of either PCV20 alone or PCV15 in series with PPSV23 for all adults aged ≥65 years, and for adults aged 19–64 years with certain underlying medical conditions or other risk factors* who have not previously received a PCV or whose previous vaccination history is unknown. ACIP employed the Evidence to Recommendation (EtR) framework,† using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE)§ approach to guide its deliberations regarding use of these vaccines. Before this, PCV13 and PPSV23 were recommended for use for U.S. adults and the recommendations varied by age and risk groups. This was simplified in the new recommendations.
PPSV23 has been recommended for use in the United States since the 1980s for adults aged ≥65 years and for younger adults with underlying conditions that increase their risk for pneumococcal disease (1). PCV13 was first recommended for use in U.S. children in 2010, and indirect effects from its use in children reduced PCV13-type pneumococcal disease incidence in all adult groups (Figure). In 2012, ACIP recommended administration of PCV13 in series with PPSV23 for adults with immunocompromising conditions,¶ cerebrospinal fluid leaks, or cochlear implants (2), and in 2014, the recommendation was extended to all adults aged ≥65 years (3). On the basis of review of accrued evidence, the PCV13 recommendation was changed in 2019 to shared clinical decision-making for adults aged ≥65 years without an immunocompromising condition, cerebrospinal fluid leak, or cochlear implant. The recommended pneumococcal vaccine doses and intervals between doses differ by age and underlying conditions, making adult pneumococcal vaccine recommendations complicated.
Recent systematic reviews continue to support the effectiveness of PCV13 against invasive pneumococcal disease (IPD)** and pneumococcal pneumonia among adults (4,5). Whereas effectiveness of PPSV23 against IPD has been demonstrated, data on effectiveness against pneumococcal pneumonia were considered to be inconsistent (3); recent observational studies reported 21%–46% effectiveness against PPSV23-type pneumococcal pneumonia when PPSV23 was given <5 years before illness onset (6–8). Nevertheless, older adults and adults with chronic medical conditions†† or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants (certain underlying conditions) remain at increased risk for pneumococcal disease, accounting for >90% of adult IPD cases in 2019 (Active Bacterial Core surveillance, unpublished data, 2021).
During February–October 2021, ACIP reviewed the epidemiology of pneumococcal disease and considerations for use of PCV15 and PCV20 in adults. The ACIP Pneumococcal Vaccines Work Group (Work Group) evaluated the quality of evidence for PCV15 and PCV20 immunogenicity and safety using the GRADE approach.§§ Using the EtR framework,¶¶ the Work Group reviewed relevant scientific evidence regarding the benefits and harms of PCV15 and PCV20 use among adults aged ≥65 years and younger adults with certain underlying conditions. Within the EtR framework, ACIP considered the importance of the public health problem, benefits and harms, target populations’ values and preferences, resource use, equity, acceptability, and feasibility for PCV15 or PCV20 use. After a systematic review of the literature, the Work Group defined critical outcomes and used GRADE to assess certainty of evidence rated on a scale of 1 (high certainty) to 4 (very low certainty) (9).
Evidence
Pneumococcal disease incidence in adults. During 2018–2019, the incidence of all IPD in adults aged ≥65 years was 24 per 100,000 population (Figure), and PCV13 serotypes accounted for 27% of cases; additional serotypes unique to PCV15,*** PCV20,††† and PPSV23§§§ caused 15%, 27%, and 35% of IPD, respectively. In adults aged 19–64 years with certain underlying conditions, PCV13 serotypes accounted for 30% of IPD; serotypes unique to PCV15, PCV20, and PPSV23 caused 13%, 28%, and 43% of IPD, respectively. Estimates of pneumococcal pneumonia incidence are more variable. Annual incidence among U.S. adults aged <65 and ≥65 years hospitalized with community-acquired pneumonia was estimated at 126–422 and 847–3,365 per 100,000, respectively, during 2010–2016 (10). In a multisite study of adults hospitalized with community-acquired pneumonia, 4.6% of cases were caused by PCV13 serotypes, and 1.4% and 3.3% were caused by additional serotypes included in PCV15 and PCV20, respectively (11).
PCV15 immunogenicity. PCV15 contains pneumococcal polysaccharide serotypes 22F and 33F in addition to the PCV13 serotypes, conjugated to CRM197 (genetically detoxified diphtheria toxin) (9). Phase II and III randomized controlled trials (RCTs) evaluated the immunogenicity and safety of a dose of PCV15 compared with a dose of PCV13 in healthy adults aged ≥50 years (12–14), adults aged 18–49 years who are Native American (a population with higher rates of IPD than the general U.S. population) (15) or with ≥1 risk condition for pneumococcal disease (16), and adults aged ≥18 years with HIV infection (17). Serotype-specific functional antibody responses were measured 1 month after vaccination using an opsonophagocytic activity (OPA) assay. Correlates of protection have not been established for adults. In one phase III RCT among adults aged ≥50 years, PCV15 met the noninferiority criteria¶¶¶ compared with PCV13 for the 13 shared serotypes and had statistically significantly greater response**** for shared serotype 3 and PCV15-unique serotypes 22F and 33F (14). In studies that evaluated the immunogenicity of PCV15 or PCV13 followed by PPSV23 2–12 months later (16–18), persons who received PCV15 had numerically similar or higher OPA geometric mean antibody titers (GMTs) for 9–13†††† shared PCV13 serotypes and a higher percentage of seroresponders§§§§ for 5–11 shared serotypes compared with persons who received PCV13 when measured 1 month after receipt of PPSV23.
PCV15 safety. Safety of PCV15 was assessed in seven RCTs with 5,630 participants aged ≥18 years who received 1 dose of PCV15. Most participants were immunocompetent; however, one study included 302 adults with HIV infection. Participants included those vaccinated with PPSV23 ≥1 year before receiving PCV15, those who received PCV15 followed by PPSV23, and those who received PCV15 concomitantly with a seasonal inactivated quadrivalent influenza vaccine (QIV). The most frequently reported adverse reactions were injection site pain, fatigue, and myalgia. The rates of serious adverse events (SAEs) within 6 months of vaccination were 2.5% among PCV15 recipients and 2.4% among PCV13 recipients. No SAEs or deaths were considered to be related to the study vaccines (9,19).
PCV20 immunogenicity. PCV20 contains pneumococcal polysaccharide serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F, in addition to PCV13 serotypes, conjugated to CRM197 (20). A phase II study among adults aged 60–64 years and two phase III RCTs among adults aged ≥18 years evaluated immunogenicity and safety of PCV20 compared with PCV13 and with PPSV23 for the seven additional serotypes included in PCV20 (21–23). These studies included adults with stable medical conditions, but none included adults with immunocompromising conditions. Compared with PCV13 recipients, PCV20 recipients elicited responses that met noninferiority criteria¶¶¶¶ for all 13 serotypes in a phase III trial among adults aged ≥60 years (21); however, PCV20 recipients appeared to have lower GMTs and included a lower percentage of seroresponders to 12–13 of the 13 PCV13-shared serotypes (21,22). Compared with PPSV23 recipients, PCV20 recipients had numerically higher GMTs and a higher percentage of seroresponders to six of seven (excluding serotype 8) shared non-PCV13 serotypes (21,23); noninferiority criteria were met for those six serotypes (21).
PCV20 safety. Safety of PCV20 was assessed in six trials among immunocompetent adults aged ≥18 years that included a total of 4,552 participants who received PCV20. Participants included those who were naïve to pneumococcal vaccination and those who had previously received pneumococcal vaccination. The most frequently reported adverse reactions were injection site pain, muscle pain, fatigue, headache, and joint pain. SAEs reported within 6 months after vaccination occurred among 1.5% of PCV20 recipients and 1.8% among controls. No SAEs or deaths were considered to be related to study vaccines (20,24).
Intervals between PCV and PPSV23. Findings from eight immunogenicity studies that evaluated the immune response after a sequence of 7-valent PCV, PCV13, or PCV15 followed by PPSV23 administered at intervals of 2, 6, or 12 months or 3–4 years were reviewed (16–18,25–29). Three studies comparing intervals ranging from 2 to 6 months between administration of PCV and PPSV23 found no significant difference in immunogenicity measured after PPSV23 receipt, although reactogenicity tended to be higher with shorter intervals (25–29). In a study that compared antibody responses to 1 dose of PCV13 with responses to PCV13 followed by PPSV23 1 year apart, the immune responses following PPSV23 were significantly lower compared with the responses after a dose of PCV13 for eight of 12 common serotypes (27). In another study that compared antibody response to 1 dose of PCV13 with responses to PCV13 followed by PPSV23 approximately 4 years apart, the immune responses following PPSV23 were significantly higher for seven of 12 common serotypes (26). These findings suggested that longer intervals between administration of PCV and PPSV23 might improve immunogenicity in immunocompetent adults, although a direct comparison between a 1- versus 4-year interval was not made.
Cost-effectiveness. Economic models assessed cost-effectiveness of the new policy options compared with existing recommendations (30). Three economic models assessed PCV20 alone for all adults aged ≥65 years; cost-effectiveness estimates ranged from cost-saving***** to $39,000 per quality-adjusted life-year (QALY) gained. Two economic models assessed use of PCV15 in series with PPSV23 for all adults aged ≥65 years; estimates ranged from cost-saving to $282,000 per QALY gained. The CDC model found cost savings in all scenarios for use of either PCV20 alone or PCV15 in series with PPSV23 for all adults aged ≥65 years. Cost estimates of policy options for adults aged 19–64 years with certain underlying medical conditions ranged from $11,000 to $292,000 per QALY gained for PCV20 and from $250,000 to $656,000 for PCV15 in series with PPSV23.
Summary. Use of PCV20 alone or PCV15 in series with PPSV23 is expected to reduce pneumococcal disease incidence in adults aged ≥65 years and in those aged 19–64 years with certain underlying conditions. Findings from studies suggested that the immunogenicity and safety of PCV20 alone or PCV15 in series with PPSV23 were comparable to PCV13 alone or PCV13 in series with PPSV23. Cost-effectiveness studies demonstrated that use of PCV20 alone or PCV15 in series with PPSV23 for adults at age 65 years was cost-saving. The new policy simplifies adult pneumococcal vaccine recommendations (Table 1) and is expected to improve vaccine coverage among adults and prevent more pneumococcal disease. An amendment to recommend PCV20 for all adults aged ≥50 years instead of age ≥65 years was considered but rejected (Table 2). A summary of Work Group deliberations on use of either PCV20 alone or PCV15 in series with PPSV23 for all adults aged ≥65 years or adults aged 19–64 years with certain underlying conditions is available in the EtR tables.
New Pneumococcal Vaccine Recommendations
Adults aged ≥65 years. Adults aged ≥65 years who have not previously received PCV or whose previous vaccination history is unknown should receive 1 dose of PCV (either PCV20 or PCV15). When PCV15 is used, it should be followed by a dose of PPSV23 (Table 1).
Adults aged 19–64 years with certain underlying medical conditions or other risk factors. Adults aged 19–64 years with certain underlying medical conditions or other risk factors who have not previously received PCV or whose previous vaccination history is unknown should receive 1 dose of PCV (either PCV20 or PCV15). When PCV15 is used, it should be followed by a dose of PPSV23.
Clinical Guidance
Dosing schedule. When PCV15 is used, the recommended interval between administration of PCV15 and PPSV23 is ≥1 year. A minimum interval of 8 weeks can be considered for adults with an immunocompromising condition, cochlear implant, or cerebrospinal fluid leak to minimize the risk for IPD caused by serotypes unique to PPSV23 in these vulnerable groups (31).
Adults with previous PPSV23 only. Adults who have only received PPSV23 may receive a PCV (either PCV20 or PCV15) ≥1 year after their last PPSV23 dose. When PCV15 is used in those with history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
Adults with previous PCV13. The incremental public health benefits of providing PCV15 or PCV20 to adults who have received PCV13 only or both PCV13 and PPSV23 have not been evaluated. These adults should complete the previously recommended PPSV23††††† series (2,30).
Coadministration with other vaccines. PCV15, PCV20, or PPSV23 can be coadministered with QIV in an adult immunization program, as concomitant administration (PCV15 or PPSV23 and QIV [Fluarix], PCV20 and adjuvanted QIV [Fluad]) has been demonstrated to be immunogenic and safe. However, slightly lower pneumococcal serotype-specific OPA GMTs or geometric mean concentrations were reported when pneumococcal vaccines were coadministered with QIV compared with when pneumococcal vaccines were given alone (9,19,32,33). Currently, no data are available on coadministration with other vaccines (e.g., tetanus, diphtheria, acellular pertussis vaccine, hepatitis B, or zoster vaccine) among adults. Evaluation of coadministration of PCV15, PCV20, or PPSV23 with COVID-19 vaccines is ongoing (34,35).
Future Research and Monitoring Priorities
CDC and ACIP will continue to assess safety of PCV15 and PCV20 vaccines, monitor the impact of implementation of new recommendations, and assess postimplementation vaccine effectiveness and update pneumococcal vaccination recommendations as appropriate.
Before administering PCV20, PCV15, or PPSV23, health care providers should consult relevant package inserts (9,20,36) regarding precautions and contraindications. Adverse events occurring after administration of any vaccine should be reported to the Vaccine Adverse Event Reporting System (VAERS). Reports can be submitted to VAERS online, by fax, or by mail. Additional information about VAERS is available at https://vaers.hhs.gov/.
Acknowledgments
Members of the Advisory Committee on Immunization Practices (member roster for August 24, 2021–June 20, 2022, is available at https://www.cdc.gov/vaccines/acip/members/index.html).
ACIP Pneumococcal Vaccines Work Group
Chair: Katherine A. Poehling, Wake Forest School of Medicine; ACIP members: Sarah S. Long, Drexel University College of Medicine; H. Keipp Talbot, Vanderbilt University Medical Center. Ex officio members: Jeffrey Kelman, Centers for Medicare & Medicaid Services; Lucia Lee, Tina Mongeau, Food and Drug Administration; Thomas Weiser, Uzo Chukwuma, Indian Health Service; Kristina Lu, Mamodikoe Makhene, National Institutes of Health; Liaison representatives: Lynn Fisher, American Academy of Family Physicians; Mark Sawyer, American Academy of Pediatrics/Committee on Infectious Diseases; Jason Goldman, American College of Physicians; David Nace, American Geriatrics Society/The Society for Post-Acute and LTC Medicine; Emily Messerli, Association of Immunization Managers; Elissa Abrams, Oliver Baclic, Canadian National Advisory Committee on Immunization; Carol Baker, Infectious Diseases Society of America; William Schaffner, National Foundation for Infectious Diseases; Virginia Cane, National Medical Association; Consultants: Doug Campos-Outcalt, University of Arizona; Monica M. Farley, Atlanta Veterans Affairs Medical Center/Emory University; Keith Klugman, Bill & Melinda Gates Foundation; Rebecca L. Morgan, McMaster University; Arthur Reingold, University of California, Berkeley; Lorry Rubin, Cohen Children’s Medical Center of Northwell Health; Cynthia Whitney, Emory University; Richard K. Zimmerman, University of Pittsburgh. Marc Fischer, Penina Haber, Pedro Moro, Sarah Schillie, CDC.
Corresponding author: Miwako Kobayashi, [email protected], 404-639-2215.
1National Center for Immunization and Respiratory Diseases, CDC; 2Epidemic Intelligence Service, CDC; 3CDC Foundation; 4University of Arizona, College of Medicine, Phoenix, Arizona; 5Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario; 6Drexel University College of Medicine, Philadelphia, Pennsylvania; 7Vanderbilt University School of Medicine, Nashville, Tennessee; 8Wake Forest School of Medicine, Winston-Salem, North Carolina.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Katherine A. Poehling reports institutional support from Safe Sleep for All Newborns, Love Out Loud Early Childhood Fellowship, Intimate Partner Violence Collaborative Project, Because You Matter: Conversations You Want about COVID-19, text messaging follow-up for patients who missed well child visits, and Reimagining Health and Wellness by Mothers for Our Babies, Families, and Communities. H. Keipp Talbott reports institutional grants from the National Institutes of Health. No other potential conflicts of interest were disclosed.
* Alcoholism; chronic heart, liver, or lung disease; chronic renal failure; cigarette smoking; cochlear implant; congenital or acquired asplenia; cerebrospinal fluid leak; diabetes mellitus; generalized malignancy; HIV; Hodgkin disease; immunodeficiency; iatrogenic immunosuppression; leukemia, lymphoma, or multiple myeloma; nephrotic syndrome; solid organ transplant; sickle cell disease; or other hemoglobinopathies.
† https://www.cdc.gov/vaccines/acip/recs/grade/downloads/acip-evidence-recs-framework.pdf
§ https://www.cdc.gov/vaccines/acip/recs/grade/about-grade.html
¶ Immunocompromising conditions are defined as chronic renal failure, nephrotic syndrome, immunodeficiency, iatrogenic immunosuppression, generalized malignancy, HIV, Hodgkin disease, leukemia, lymphoma, multiple myeloma, solid organ transplant, congenital or acquired asplenia, sickle cell disease, or other hemoglobinopathies.
** The case definition used by CDC’s Active Bacterial Core surveillance is isolation of S. pneumoniae from a normally sterile site or pathogen-specific nucleic acid in a specimen obtained from a normally sterile body site using a validated molecular test. https://www.cdc.gov/abcs/methodology/case-def-ascertain.html
†† Alcoholism; chronic heart, liver, or lung disease; cigarette smoking; or diabetes mellitus.
§§ https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV20-risk-based.html; https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV15-PPSV23-risk-based.html; https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV20-age-based.html; https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV15-PPSV23-age-based.html
¶¶ https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV20-risk-based-etr.html; https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV20-age-based-etr.html; https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV15-PPSV23-risk-based-etr.html; https://www.cdc.gov/vaccines/acip/recs/grade/pneumo-PCV15-PPSV23-age-based-etr.html
*** Serotypes 22F and 33F, in addition to PCV13 serotypes.
††† Serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F, in addition to PCV13 serotypes.
§§§ Serotypes 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F, in addition to PCV13 serotypes.
¶¶¶ Lower bound of the two-sided 95% CI of the OPA GMT ratio (PCV15 / PCV13) to be >0.5.
**** For PCV15-unique serotypes 22F and 33F, defined as the lower bound of the two-sided 95% CI of the OPA GMT ratio (V114 / PCV13) to be >2.0 and the lower bound of the two-sided 95% CI of the differences (V114 − PCV13) between the percentages of participants with a fourfold rise to be >0.1. For serotype 3, defined as the lower bound of the two-sided 95% CI of the OPA GMT ratio (V114 / PCV13) to be >1.2 and the lower bound of the two-sided 95% CI of the differences (V114 − PCV13) between the percentages of participants with a fourfold rise to be >0.
†††† Range reflects the difference in results across studies.
§§§§ Subjects with a fourfold or larger rise in OPA GMT titer postvaccination compared with prevaccination.
¶¶¶¶ Defined as the lower bound of the two-sided 95% CI of the ratio (PCV20 / PCV13) of opsonophagocytic geometric mean titers being >0.5.
***** Lower cost and improved health outcomes compared with previous recommendations.
††††† For adults who have received PCV13 but have not completed their recommended pneumococcal vaccine series with PPSV23, one dose of PCV20 may be used if PPSV23 is not available.
References
- CDC; Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010;59:1102–6. PMID:20814406
- CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9. PMID:23051612
- Tomczyk S, Bennett NM, Stoecker C, et al.; CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5. PMID:25233284
- Childs L, Kobayashi M, Farrar JL, Pilishvili T. The efficacy and effectiveness of pneumococcal vaccines against pneumococcal pneumonia among adults: a systematic review and meta-analysis. Open Forum Infect Dis 2021;8(Supp 1):S130–1. https://doi.org/10.1093/ofid/ofab466.215
- Farrar JL, Kobayashi M, Childs L, Pilishvili T. Systematic review and meta-analysis of pneumococcal vaccine effectiveness against invasive pneumococcal disease among adults. Open Forum Infect Dis 2021;8(Supp 1):S134–5. https://doi.org/10.1093/ofid/ofab466.223
- Suzuki M, Dhoubhadel BG, Ishifuji T, et al.; Adult Pneumonia Study Group-Japan (APSG-J). Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis 2017;17:313–21. https://doi.org/10.1016/S1473-3099(17)30049-X PMID:28126327
- Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med 2020;17:e1003326. https://doi.org/10.1371/journal.pmed.1003326 PMID:33095759
- Kim JH, Chun BC, Song JY, et al. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: a case-control study. Vaccine 2019;37:2797–804. https://doi.org/10.1016/j.vaccine.2019.04.017 PMID:31005428
- VAXNEUVANCE. Package insert. Silver Spring, MD: Food and Drug Administration; 2021. https://www.fda.gov/media/150819/download
- McLaughlin JM, Khan FL, Thoburn EA, Isturiz RE, Swerdlow DL. Rates of hospitalization for community-acquired pneumonia among US adults: a systematic review. Vaccine 2020;38:741–51. https://doi.org/10.1016/j.vaccine.2019.10.101 PMID:31843272
- Isturiz R, Grant L, Gray S, et al. Expanded analysis of 20 pneumococcal serotypes associated with radiographically confirmed community-acquired pneumonia in hospitalized US adults. Clin Infect Dis 2021;73:1216–22. https://doi.org/10.1093/cid/ciab375 PMID:33982098
- Ermlich SJ, Andrews CP, Folkerth S, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naïve adults ≥50 years of age. Vaccine 2018;36:6875–82. https://doi.org/10.1016/j.vaccine.2018.03.012 PMID:29559167
- Peterson JT, Stacey HL, MacNair JE, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine compared to 13-valent pneumococcal conjugate vaccine in adults ≥65 years of age previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Hum Vaccin Immunother 2019;15:540–8. https://doi.org/10.1080/21645515.2018.1532250 PMID:30427749
- Platt HL, Cardona JF, Haranaka M, et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults 50 years of age and older (PNEU-AGE). Vaccine 2022;40:162–72. https://doi.org/10.1016/j.vaccine.2021.08.049 PMID:34507861
- Said MA, O’Brien KL, Nuorti JP, Singleton R, Whitney CG, Hennessy TW. The epidemiologic evidence underlying recommendations for use of pneumococcal polysaccharide vaccine among American Indian and Alaska Native populations. Vaccine 2011;29:5355–62. https://doi.org/10.1016/j.vaccine.2011.05.086 PMID:21664217
- Merck Sharp & Dohme Corp. A study to evaluate the safety, tolerability, and immunogenicity of V114 followed by PNEUMOVAX™23 in adults at increased risk for pneumococcal disease (V114–017/PNEU-DAY). Bethesda, MD: US National Library of Medicine; 2021. https://ClinicalTrials.gov/show/NCT03547167
- Mohapi L, Pinedo Y, Osiyemi O, et al. Safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, in adults living with HIV: a randomized phase 3 study. AIDS 2021. Epub November 22, 2021. https://journals.lww.com/aidsonline/Abstract/9000/Safety_and_immunogenicity_of_V114,_a_15_valent.96271.aspx
- Song JY, Chang CJ, Andrews C, et al.; V114-016 (PNEU-PATH) study group. Safety, tolerability, and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, followed by sequential PPSV23 vaccination in healthy adults aged ≥50 years: a randomized phase III trial (PNEU-PATH). Vaccine 2021;39:6422–36. https://doi.org/10.1016/j.vaccine.2021.08.038 PMID:34489128
- Food and Drug Administration. Summary basis for regulatory action—VAXNEUVANCE. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/151201/download
- PREVNAR 20. Package insert. Silver Spring, MD: Food and Drug Administration; 2021. https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20
- Essink B, Sabharwal C, Xu X, et al. 3. Phase 3 pivotal evaluation of 20-valent pneumococcal conjugate vaccine (PCV20) safety, tolerability, and immunologic noninferiority in participants 18 years and older. Open Forum Infect Dis 2020;7(Supplement_1):S2. https://doi.org/10.1093/ofid/ofaa417.002
- Klein NP, Peyrani P, Yacisin K, et al. A phase 3, randomized, double-blind study to evaluate the immunogenicity and safety of 3 lots of 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults 18 through 49 years of age. Vaccine 2021;39:5428–35. https://doi.org/10.1016/j.vaccine.2021.07.004 PMID:34315611
- Hurley D, Griffin C, Young M Jr, et al. Safety, tolerability, and immunogenicity of a 20–valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis 2021;73:e1489–97. https://doi.org/10.1093/cid/ciaa1045 PMID:32716500
- Food and Drug Administration. Summary basis for regulatory action—PREVNAR20. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration, 2021. https://www.fda.gov/media/150388/download
- Miernyk KM, Butler JC, Bulkow LR, et al. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in Alaska Native adults 55–70 years of age. Clin Infect Dis 2009;49:241–8. https://doi.org/10.1086/599824 PMID:19522655
- Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013;31:3594–602. https://doi.org/10.1016/j.vaccine.2013.04.084 PMID:23688525
- Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60–64 years of age. Vaccine 2014;32:2364–74. https://doi.org/10.1016/j.vaccine.2014.02.002 PMID:24606865
- Buchwald UK, Andrews CP, Ervin J, et al.; V110–029 Study Group. Sequential administration of Prevnar 13™ and PNEUMOVAX™ 23 in healthy participants 50 years of age and older. Hum Vaccin Immunother 2021;17:2678–90. https://doi.org/10.1080/21645515.2021.1888621 PMID:34019468
- Nguyen MTT, Lindegaard H, Hendricks O, Jørgensen CS, Kantsø B, Friis-Møller N. Initial serological response after prime-boost pneumococcal vaccination in rheumatoid arthritis patients: results of a randomized controlled trial. J Rheumatol 2017;44:1794–803. https://doi.org/10.3899/jrheum.161407 PMID:28966211
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019;68:1069–75. https://doi.org/10.15585/mmwr.mm6846a5 PMID:31751323
- Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7. https://doi.org/10.15585/mmwr.mm6434a4 PMID:26334788
- Pfizer. Safety and immunogenicity of 20vPnC coadministered with SIIV in adults ≥65 years of age. Bethesda, MD: US National Library of Medicine; 2020. https://ClinicalTrials.gov/show/NCT04526574
- Ofori-Anyinam O, Leroux-Roels G, Drame M, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine co-administered with a 23-valent pneumococcal polysaccharide vaccine versus separate administration, in adults ≥50 years of age: results from a phase III, randomized, non-inferiority trial. Vaccine 2017;35:6321–8. https://doi.org/10.1016/j.vaccine.2017.09.012 PMID:28987445
- Pfizer. Safety and immunogenicity study of 20vPnC when coadministered with a booster dose of BNT162b2. Bethesda, MD: US National Library of Medicine; 2021. https://clinicaltrials.gov/ct2/show/NCT04887948
- Merck Sharp & Dohme Corp. Safety, tolerability, and immunogenicity of V110 or V114 co-administered with a booster dose of mRNA-1273 in healthy adults (V110-911). Bethesda, MD: US National Library of Medicine; 2021. https://ClinicalTrials.gov/show/NCT05158140
- Food and Drug Administration. PNEUMOVAX23. Package insert. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/pneumovax-23-pneumococcal-vaccine-polyvalent
 FIGURE. Incidence of all invasive pneumococcal disease and 13-valent pneumococcal conjugate vaccine-type* invasive pneumococcal disease among adults aged ≥19 years, by invasive pneumococcal disease type and age group — United States, 2007–2019†
FIGURE. Incidence of all invasive pneumococcal disease and 13-valent pneumococcal conjugate vaccine-type* invasive pneumococcal disease among adults aged ≥19 years, by invasive pneumococcal disease type and age group — United States, 2007–2019†
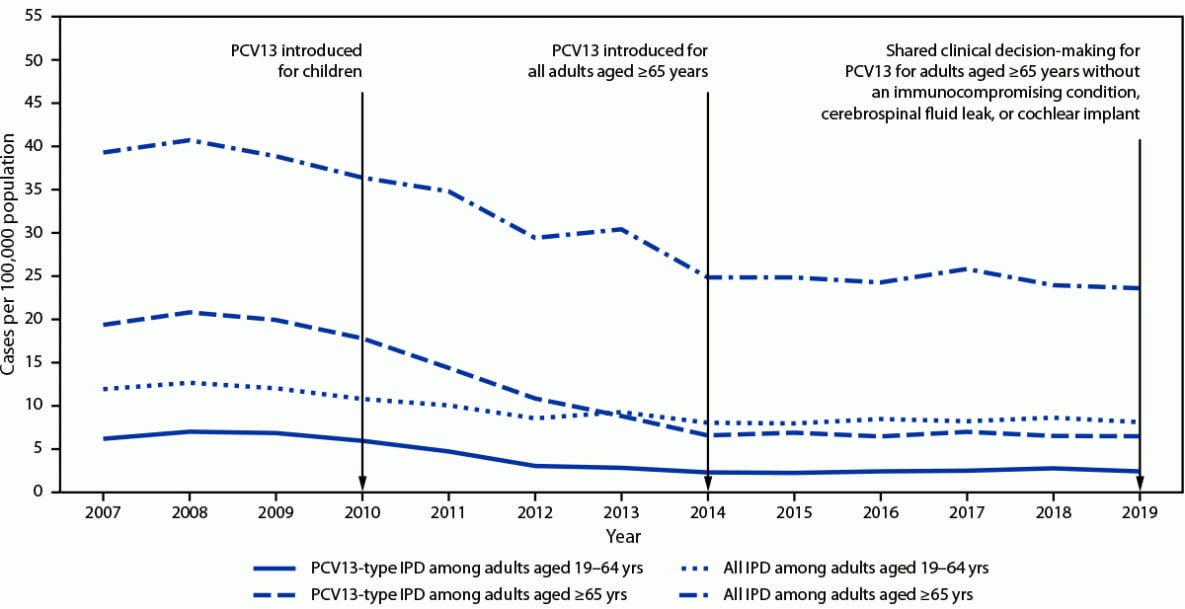
Abbreviations: IPD = invasive pneumococcal disease; PCV13 = 13-valent pneumococcal conjugate vaccine.
* Includes serotype 6C, which shows cross-protection from 6A antigen in PCV13 and was grouped with PCV13 serotypes for IPD incidence.
† Active Bacterial Core surveillance, 2021.
Abbreviations: CSF = cerebrospinal fluid; PCV15 =15-valent pneumococcal conjugate vaccine; PCV20 = 20-valent pneumococcal conjugate vaccine; PPSV23 = 23-valent pneumococcal polysaccharide vaccine.
* Adults with immunocompromising conditions, cochlear implant, or CSF leak might benefit from shorter intervals such as ≥8 weeks. These vaccine doses do not need to be repeated if given before age 65 years.
† Includes congestive heart failure and cardiomyopathies.
§ Adults with immunocompromising conditions, cochlear implant, or CSF leak might benefit from shorter intervals such as ≥8 weeks.
¶ Includes chronic obstructive pulmonary disease, emphysema, and asthma.
** Indicates immunocompromising conditions.
†† Includes B- (humoral) or T-lymphocyte deficiency, complement deficiencies (particularly C1, C2, C3, and C4 deficiencies), and phagocytic disorders (excluding chronic granulomatous disease).
§§ Diseases requiring treatment with immunosuppressive drugs, including long-term systemic corticosteroids and radiation therapy.
Abbreviations: ACIP = Advisory Committee on Immunization Practices; PCV = pneumococcal conjugate vaccine ; PCV15 = 15-valent PCV; PCV20 = 20-valent PCV; PPSV23 = 23-valent pneumococcal polysaccharide vaccine.
Suggested citation for this article: Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:109–117. DOI: http://dx.doi.org/10.15585/mmwr.mm7104a1.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to [email protected].