ENGINI: Empowering Next Generation Implantable Neural Interfaces
Completed Project (2015-2020)
EPSRC Early Career Research Fellow: Timothy Constandinou
Research Team: Nur Ahmadi, Matthew Cavuto, Peilong Feng, Lieuwe Leene, Michal Maslik, Federico Mazza, Oscar Savolainen, Katarzyna Szostak
Collaborators: Andrew Jackson (Newcastle), Jinendra Ekanayake (UCL), Maysam Ghovanloo (GeorgiaTech), Andrew Mason (Michigan State University), Nick Donaldson (UCL)
Funding: Engineering and Physical Sciences Research Council (EPSRC) EP/M020975/1
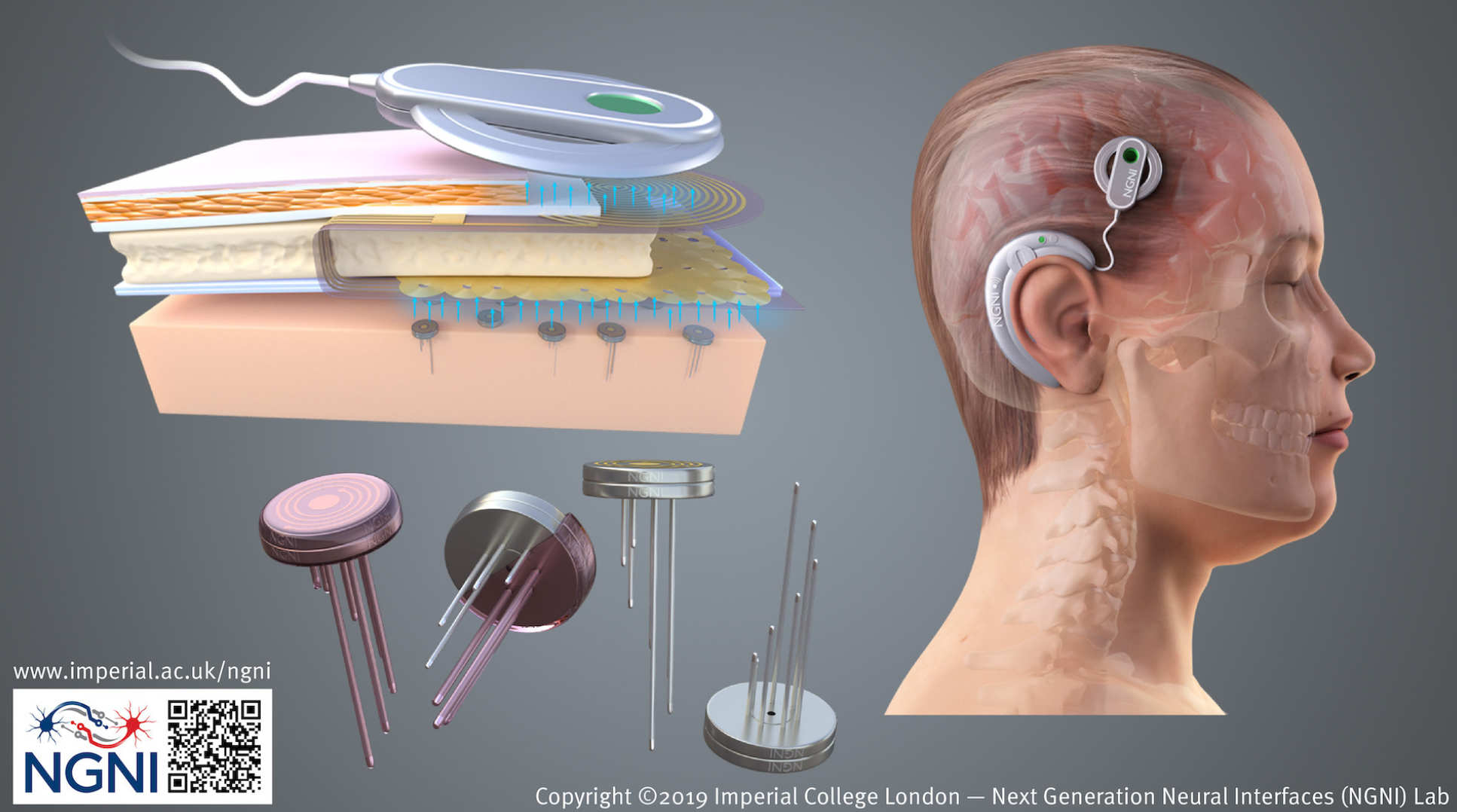
Being able to control devices with our thoughts is a concept that has for long captured the imagination. Neural Interfaces or Brain Machine Interfaces (BMIs) are devices that aim to do precisely this. Next-generation devices will be distributed like the brain itself. It is currently estimated that if we were able to record electrical activity simultaneously from between 1,000 and 10,000 neurons, this would enable useful prosthetic control (e.g. of a prosthetic arm).
However, rather than relying on a single, highly complex implant and trying to cram more and more channels in this (the current paradigm), the idea here is to develop a simpler, smaller, well-engineered primitive and deploy multiple such devices. These must be each compact, autonomous, calibration-free, and completely wireless. It is envisaged that each device will be mm-scale, and be capable of recording only a few channels (i.e. up to 20), but also perform real-time signal processing.
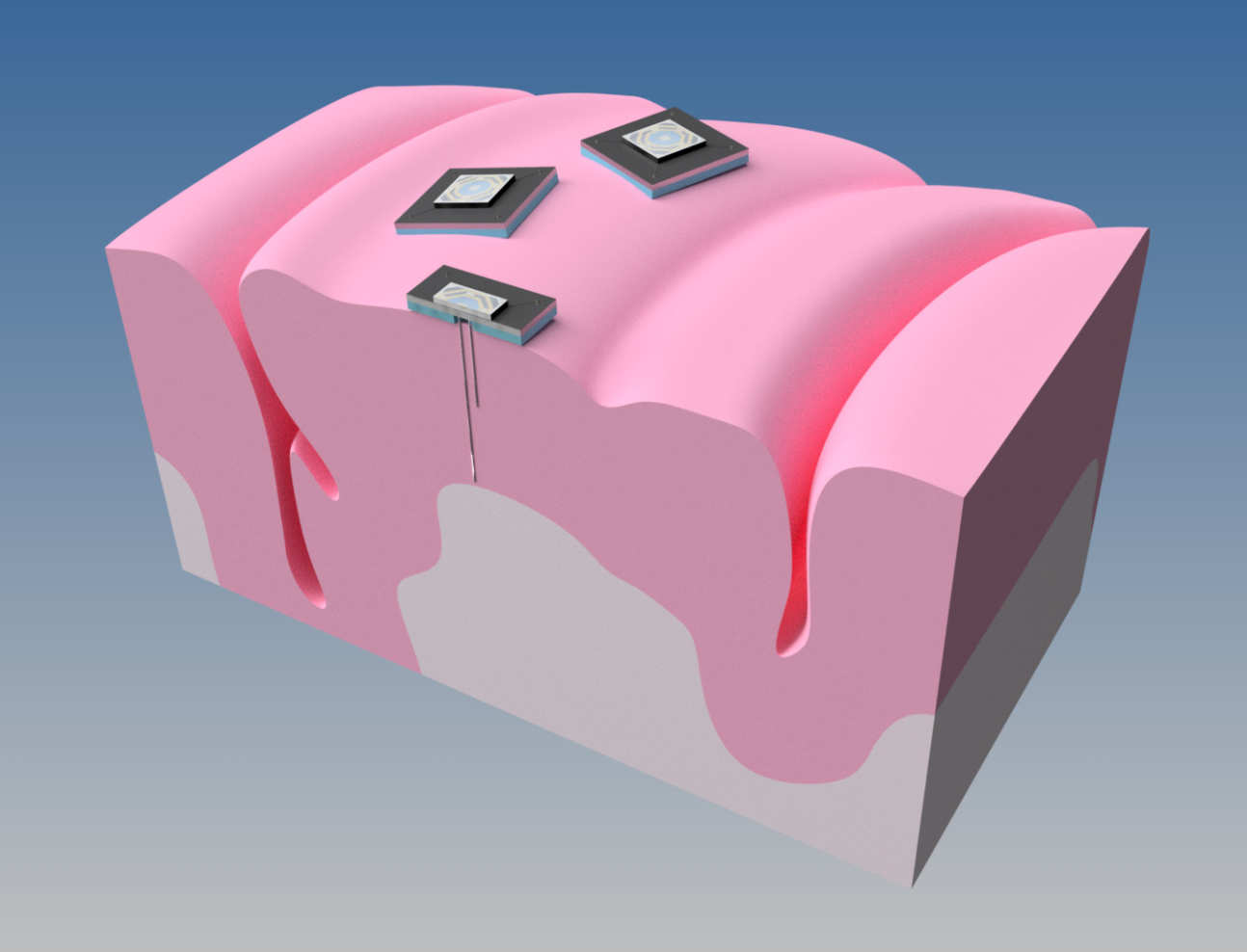 This processing will achieve data reduction to wirelessly communicate only useful information, rather than raw data, which can most often be just noise and of no use. Making these underlying devices "simpler" will overcome many of the common challenges that are associated with scaling of neural interfaces, for example, wires breaking, biocompatibility of the packaging, thermal dissipation, and yield.
This processing will achieve data reduction to wirelessly communicate only useful information, rather than raw data, which can most often be just noise and of no use. Making these underlying devices "simpler" will overcome many of the common challenges that are associated with scaling of neural interfaces, for example, wires breaking, biocompatibility of the packaging, thermal dissipation, and yield.
By distributing tens to hundreds of these in a "grid" of neural interfaces, many of the desirable features of distributed networks come into play; for example, redundancy and robustness to single component failure.
The first tangible application for this platform will see several such devices implanted as freely floating mm-scale probes for recording from the cortex. These will communicate the neural "control signals" to an external prosthetic device. These can then, for example, be used for an amputee to control a robotic prosthetic; a paraplegic to control a mobility aid; or an individual with locked-in syndrome to communicate with the outside world.
Publications
Publications
2023
- A. Meimandi, P. Feng, M. Carminati, T. G. Constandinou, and S. Carrara, “Implantable biosensor for brain dopamine using microwire-based electrodes,” in 2023 IEEE BioSensors Conference (BioSensors), pp. 1–4, 2023. doi: https://doi.org/10.1109/BioSensors58001.2023.10280954
- S. Khan, W. Anderson, and T. G. Constandinou, “Surgical implantation of brain computer interfaces,” JAMA Surgery, 2023. doi: https://doi.org/10.1001/jamasurg.2023.2399
- Z. Zhang, P. Feng, A. Oprea, and T. G. Constandinou, “Calibration-free and hardware-efficient neural spike detection for brain machine interfaces,” IEEE Transactions on Biomedical Circuits and Systems, vol. 17, no. 4, pp. 725–740, 2023. doi: https://doi.org/10.1109/TBCAS.2023.3278531
- Z. Zhang and T. Constandinou, “Firing-rate-modulated spike detection and neural decoding co-design,” Journal of Neural Engineering, 2023. doi: https://doi.org/10.1088/1741-2552/accece
2022
- A. Jaccottet, P. Feng, K. M. Szostak-Lipowicz, L. Keeble, and T. G. Constandinou, “Towards a hermetically micropackaged implantable wireless humidity sensor,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, 2022. doi: https://doi.org/10.1109/BioCAS54905.2022.9948556
- O. W. Savolainen, Z. Zhang, P. Feng, and T. G. Constandinou, “Hardware-efficient compression of neural multi-unit activity,” IEEE Access, pp. 1–15, 2022. doi: https://doi.org/10.1109/ACCESS.2022.3219441
- Z. Zhang and T. G. Constandinou, “Selecting an effective amplitude threshold for neural spike detection,” in 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 2022. doi: https://doi.org/10.1101/2022.01.25.477685
- N. Ahmadi, T. Adiono, A. Purwarianti, T. G. Constandinou, and C.-S. Bouganis, “Improved spike-based brain-machine interface using bayesian adaptive kernel smoother and deep learning,” IEEE Access, vol. 10, pp. 29341–29356, 2022. doi: https://doi.org/10.1109/ACCESS.2022.3159225
- Z. Zhang, O. W. Savolainen, and T. Constandinou, “Algorithm and hardware considerations for real-time neural signal on-implant processing,” Journal of Neural Engineering, vol. 19, no. 1, p. 016029, 2022. doi: https://doi.org/10.1088/1741-2552/ac5268
2021
- S. Yilmaz, T. G. Constandinou, and S. Carrara, “Integrated potentiostat design for neurotransmitter detection in wireless implants,” in IEEE Midwest Circuits and Systems (MWCAS) Conference, IEEE, 2021. doi: https://doi.org/10.1109/MWSCAS47672.2021.9531719
- O. W. Savolainen and T. G. Constandinou, “Investigating the effects of macaque primary motor cortex multi-unit activity binning period on behavioural decoding performance,” in IEEE/EMBS Conference on Neural Engineering, 2021. doi: https://doi.org/10.1109/NER49283.2021.9441197
- Z. Zhang and T. G. Constandinou, “A robust and automated algorithm that uses single-channel spike sorting to label multi-channel neuropixels data,” in IEEE/EMBS Conference on Neural Engineering, 2021. doi: https://doi.org/10.1109/NER49283.2021.9441234
- P. Feng and T. G. Constandinou, “Autonomous wireless system for robust and efficient inductive power transfer to multi-node implants,” in IEEE International Symposium on Circuits and Systems (ISCAS), 2021. doi: https://doi.org/10.1109/ISCAS51556.2021.9401594
- A. B. Rapeaux and T. G. Constandinou, “Implantable brain machine interfaces: First-in-human studies, technology challenges and trends,” Current Opinion in Biotechnology, vol. 72, pp. 102–111, 2021. doi: https://doi.org/10.1016/j.copbio.2021.10.001
- N. Ahmadi, T. G. Constandinou, and C.-S. Bouganis, “Inferring entire spiking activity from local field potentials,” Scientific Reports, vol. 11, p. 19045, 2021. doi: https://doi.org/10.1038/s41598-021-98021-9
- K. M. Szostak, M. Keshavarz, and T. G. Constandinou, “Hermetic chip-scale packaging using Au:Sn eutectic bonding for implantable devices,” Journal of Micromechanics and Microengineering, vol. 31, no. 9, p. 095003, 2021. doi: https://doi.org/10.1088/1361-6439/ac12a1
- Z. Zhang and T. G. Constandinou, “Adaptive spike detection and hardware optimization towards autonomous, high-channel-count BMIs,” Journal of Neuroscience Methods, p. 109103, 2021. doi: https://doi.org/10.1016/j.jneumeth.2021.109103
- N. Ahmadi, T. G. Constandinou, and C.-S. Bouganis, “Impact of referencing scheme on decoding performance of LFP-based brain-machine interface,” Journal of Neural Engineering, vol. 18, p. 016028, Feb 2021. doi: https://doi.org/10.1088/1741-2552/abce3c
- N. Ahmadi, T. Constandinou, and C.-S. Bouganis, “Robust and accurate decoding of hand kinematics from entire spiking activity using deep learning,” Journal of Neural Engineering, vol. 18, no. 2, p. 026011, 2021. doi: https://doi.org/10.1088/1741-2552/abde8a
2020
- O. W. Savolainen and T. G. Constandinou, “Predicting single-unit activity from local field potentials with LSTMs,” in 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), pp. 884–887, IEEE, 2020. doi: https://doi.org/10.1109/EMBC44109.2020.9175265
- O. W. Savolainen and T. G. Constandinou, “Lossless compression of intracortical extracellular neural recordings using non-adaptive huffman encoding,” in 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), pp. 4318–4321, IEEE, 2020. doi: https://doi.org/10.1109/EMBC44109.2020.9176352
2019
- N. Ahmadi, T. G. Constandinou, and C.-S. Bouganis, “End-to-end hand kinematics decoding from local field potentials using temporal convolutional network,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, 2019.
- P. Feng, M. Maslik, and T. G. Constandinou, “EM-lens enhanced power transfer and multi-node data transmission for implantable medical devices,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, 2019. doi: https://doi.org/10.1109/BIOCAS.2019.8919131
- M. Cavuto, R. Hallam, A. Rapeaux, M. Maslik, F. Troiani, and T. G. Constandinou, “Live demonstration: A public engagement platform for invasive neural interfaces,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, 2019. doi: https://doi.org/10.1109/BIOCAS.2019.8919063
- L. B. Leene and T. G. Constandinou, “A 3rd order time domain delta sigma modulator with extended-phase detection,” in 2019 IEEE International Symposium on Circuits and Systems (ISCAS), pp. 1–5, 2019. doi: https://doi.org/10.1109/ISCAS.2019.8702705
- L. B. Leene, S. Letchumanan, and T. G. Constandinou, “A 68 μW 31 kS/s fully-capacitive noise-shaping SAR ADC with 102 dB SNDR,” in 2019 IEEE International Symposium on Circuits and Systems (ISCAS), pp. 1–5, 2019. doi: https://doi.org/10.1109/ISCAS.2019.8702504
- N. Ahmadi, M. L. Cavuto, P. Feng, L. B. Leene, M. Maslik, F. Mazza, O. Savolainen, K. M. Szostak, C.-S. Bouganis, J. Ekanayake, A. Jackson, and T. G. Constandinou, “Towards a distributed, chronically-implantable neural interface,” in IEEE/EMBS Conference on Neural Engineering, 2019. doi: https://doi.org/10.1109/NER.2019.8716998
- M. L. Cavuto and T. G. Constandinou, “Investigation of insertion method to achieve chronic recording stability of a semi-rigid implantable neural probe,” in IEEE/EMBS Conference on Neural Engineering, 2019. doi: https://doi.org/10.1109/NER.2019.8717128
- N. Ahmadi, T. G. Constandinou, and C.-S. Bouganis, “Decoding hand kinematics from local field potentials using long short-term memory (LSTM) network,” in IEEE/EMBS Conference on Neural Engineering, 2019. doi: https://doi.org/10.1109/NER.2019.8717045
2018
- L. Leene and T. G. Constandinou, “Direct digital wavelet synthesis for embedded biomedical microsys- tems,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, pp. 77–80, 2018. doi: https://doi.org/10.1109/BIOCAS.2018.8584787
- P. Feng and T. G. Constandinou, “Robust wireless power transfer to multiple mm-scale freely-positioned neural implants,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, pp. 363–366, 2018. doi: https://doi.org/10.1109/BIOCAS.2018.8584730
- F. Mazza, Y. Liu, N. Donaldson, and T. G. Constandinou, “Integrated devices for micro-package integrity monitoring in mm-scale neural implants,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, pp. 295–298, 2018. doi: https://doi.org/10.1109/BIOCAS.2018.8584761
- N. Ahmadi, C.-S. Bouganis, and T. G. Constandinou, “Spike rate estimation using bayesian adaptive kernel smoother (BAKS) and its application to brain machine interfaces,” in 40th International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2018. doi: https://doi.org/10.1109/EMBC.2018.8512830
- K. Szostak and T. G. Constandinou, “Hermetic packaging for implantable microsystems: Effectiveness of sequentially electroplated AuSn alloy,” in 40th International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2018. doi: https://doi.org/10.1109/EMBC.2018.8513272
- L. Leene, M. Maslik, P. Feng, K. Szostak, F. Mazza, and T. G. Constandinou, “Autonomous SoC for neural local field potential recording in mm-scale wireless implants,” in IEEE International Symposium on Circuits and Systems (ISCAS), pp. 1–5, 2018. doi: https://doi.org/10.1109/ISCAS.2018.8351147
- N. Ahmadi, T. G. Constandinou, and C.-S. Bouganis, “Estimation of neuronal firing rate using bayesian adaptive kernel smoother (BAKS),” PLOS ONE, pp. 1–31, 2018. doi: https://doi.org/10.1371/journal.pone.0206794
- P. Feng, P. Yeon, Y. Cheng, M. Ghovanloo, and T. G. Constandinou, “Chip-scale coils for millimeter-sized bio-implants,” IEEE Transactions on Biomedical Circuits and Systems, vol. 12, no. 5, pp. 1088–1099, 2018. doi: https://doi.org/10.1109/TBCAS.2018.2853670
- L. Leene and T. G. Constandinou, “A 0.006mm2 1.2μW analogue-to-time converter for asynchronous bio-sensors,” IEEE Journal of Solid-State Circuits, vol. 53, no. 9, pp. 2604–2613, 2018. doi: https://doi.org/10.1109/JSSC.2018.2850918
- M. L. Cavuto, A. G. Winter, and T. G. Constandinou, “Apparatus and method for inserting electrode-based probes into biological tissue,” patent US20210338127A1, 2018.
- M. Maslik, Y. Liu, T. S. Lande, and T. G. Constandinou, “Continuous-time acquisition of biosignals using a charge-based ADC topology,” IEEE Transactions in Biomedical Circuits and Systems, vol. 12, no. 3, pp. 471–482, 2018. doi: https://doi.org/10.1109/TBCAS.2018.2817180
- Y. Liu, J. a. Pereira, and T. G. Constandinou, “Event-driven processing for hardware-efficient neural spike sorting,” Journal of Neural Engineering, vol. 15, no. 1, pp. 1–14, 2018. doi: https://doi.org/10.1088/1741-2552/aa9124
2017
- K. Szostak, F. Mazza, M. Maslik, L. Leene, P. Feng, and T. G. Constandinou, “Microwire-CMOS integration of mm-scale neural probes for chronic local field potential recording,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, pp. 492–495, 2017. doi: https://doi.org/10.1109/BIOCAS.2017.8325185
- L. B. Leene and T. G. Constandinou, “A 0.5V time-domain instrumentation circuit with clocked and unclocked ∆Σ operation,” in IEEE International Symposium on Circuits and Systems (ISCAS), pp. 2619– 2622, 2017. doi: https://doi.org/10.1109/ISCAS.2017.8050956
- M. Maslik, Y. Liu, T. S. Lande, and T. G. Constandinou, “A charge-based ultra-low power continuous-time ADC for data driven neural spike processing,” in IEEE International Symposium on Circuits and Systems (ISCAS), pp. 1420–1423, 2017. doi: https://doi.org/10.1109/ISCAS.2017.8050620
- P. Feng, T. G. Constandinou, P. Yeon, and M. Ghovanloo, “Millimeter-scale integrated and wirewound coils for powering implantable neural microsystems,” in IEEE Biomedical Circuits and Systems (BioCAS) Conference, pp. 488–491, 2017. doi: https://doi.org/10.1109/BIOCAS.2017.8325184
- K. M. Szostak, L. Grand, and T. G. Constandinou, “Neural interfaces for intracortical recording: Requirements, fabrication methods, and characteristics,” Frontiers in Neuroscience, vol. 11, no. 665, pp. 1–27, 2017. doi: https://doi.org/10.3389/fnins.2017.00665
- L. Leene and T. G. Constandinou, “Time domain processing techniques using ring oscillator based filter structures,” IEEE Transactions in Circuits and Systems I: Regular Papers, vol. 64, no. 12, pp. 3003–3012, 2017. doi: https://doi.org/10.1109/TCSI.2017.2715885
- L. Leene and T. G. Constandinou, “A 0.016 mm2 12b ∆ΣSAR with 14fJ/conv. for ultra low power biosensor arrays,” IEEE Transactions in Circuits and Systems I: Regular Papers, vol. 64, no. 10, pp. 2655–2665, 2017. doi: https://doi.org/10.1109/TCSI.2017.2703580
2016
- T. G. Constandinou and A. Jackson, “Implantable neural interface,” patents: US11589790B2, EP3777965A1, 2016.


