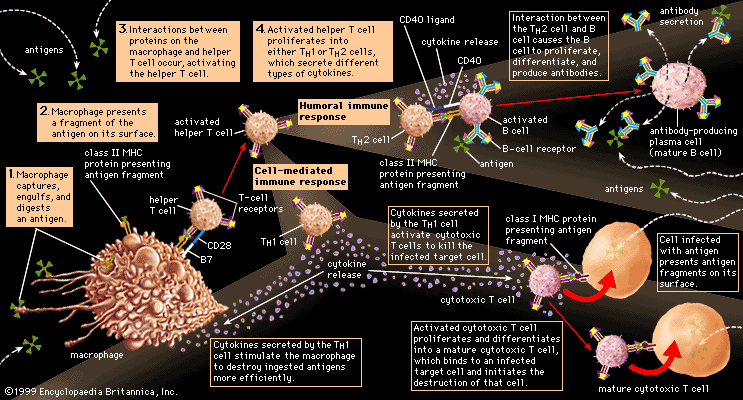
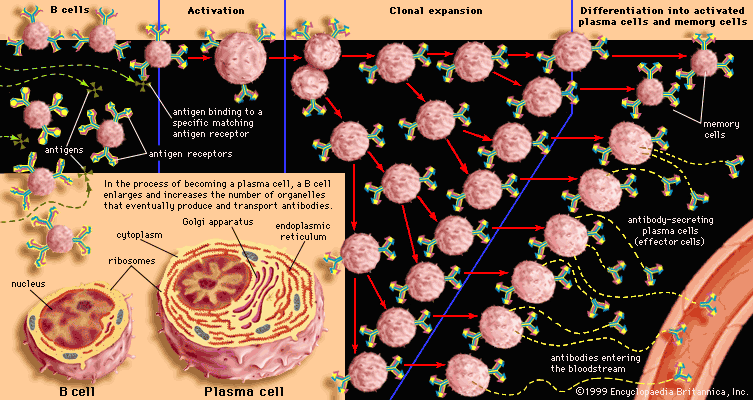
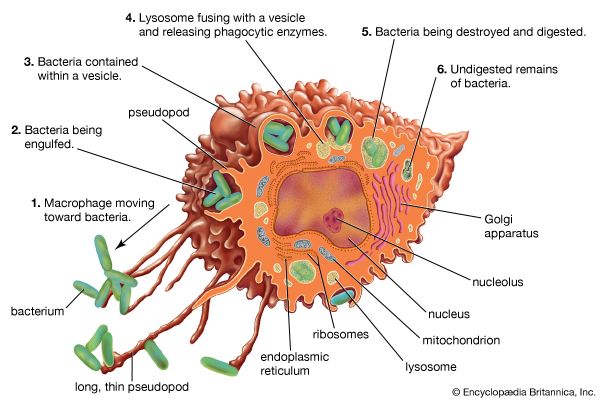
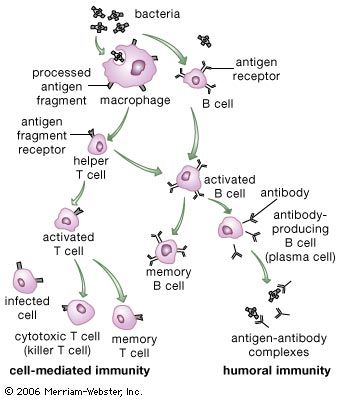
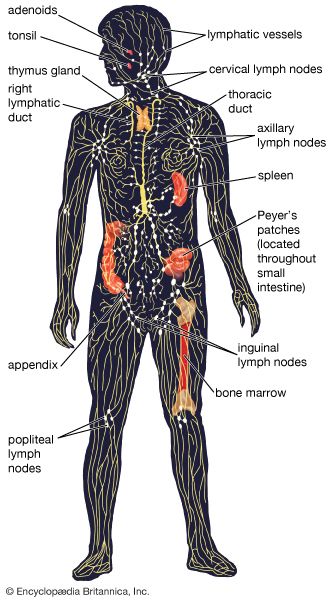
Antibody-mediated immune mechanisms
Protective attachment to antigens
Many pathogenic microorganisms and toxins can be rendered harmless by the simple attachment of antibodies. For example, some harmful bacteria, such as those that cause diphtheria and tetanus, release toxins that poison essential body cells. Antibodies, especially IgG, that combine with such toxins neutralize them. Also susceptible to simple antibody attachment are the many infectious microbes—including all viruses and some bacteria and protozoans—that live within the body cells. These pathogens bear special molecules that they use to attach themselves to the host cells so that they can penetrate and invade them. Antibodies can bind to these molecules to prevent invasion. Antibody attachment also can immobilize bacteria and protozoans that swim by means of whiplike flagella. In these instances antibodies protect simply by combining with the repeating protein units that make up these structures, although they do not kill or dispose of the microbes. The actual destruction of microbes involves phagocytosis by granulocytes and macrophages, and this is greatly facilitated by the participation of the complement system.
Activation of the complement system
Complement is a term used to denote a group of more than 30 proteins that act in concert to enhance the actions of other defense mechanisms of the body. Complement proteins are produced by liver cells and, in many tissues, by macrophages. Most of these proteins circulate in the blood and other body fluids in an inactive form. They become activated in sequential fashion; once the first protein in the pathway is turned on, the following complement proteins are called into action, with each protein turning on the next one in line.
The action of complement is nonspecific—i.e., complement proteins are not recognized by and do not interact with antigen-binding sites. In fact, complement proteins probably evolved before antibodies. Complement functions are similar among many species, and corresponding components from one species can carry out the same functions when introduced into another species. The complement system is ingenious in providing a way for antibodies, whatever their specificity, to produce the same biological effects when they combine with antigens.
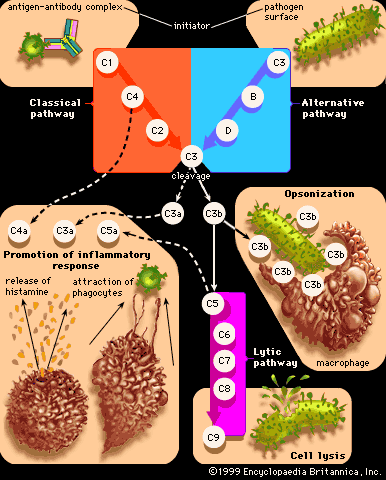
Originally immunologists thought that the complement system was initiated only by antigen-antibody complexes, but later evidence showed that other substances, such as the surface components of a microorganism alone, could trigger complement activation. Thus, there are two complement activation pathways: the first one to be discovered, the classical pathway, which is initiated by antigen-antibody complexes; and the alternative pathway, which is triggered by other means, including invading pathogens or tumour cells. (The term alternative is something of a misnomer because this pathway almost certainly evolved before the classical pathway. The terminology reflects the order of discovery, not the evolutionary age of the pathways.) The classical and alternative pathways are composed of different proteins in the first part of their cascades, but eventually both pathways converge to activate the same complement components, which destroy and eliminate invading pathogens.
The classical complement pathway is activated most effectively by IgM and the most abundant of the immunoglobulins, IgG. But, for activation to occur, antibodies must be bound to antigens (the antigen-antibody complex mentioned above). Free antibodies do not activate complement. To initiate the cascade, the first complement protein in the pathway, C1, must interact with a bound immunoglobulin. Specifically, C1 interacts with the tail of the Y portion of the bound antibody molecule—i.e., the nonspecific part of the antibody that does not bind antigen. Once bound to the antibody, C1 is cleaved, a process that activates C1 and allows it to split and activate the next complement component in the series. This process is repeated on the following proteins in the pathway until the complement protein C3—the most abundant and biologically the most important component of the complement system—is activated. The classical and alternative complement pathways converge here, at the cleavage of the C3 molecule, which, once split, produces C3a and the large active form of C3, the fragment called C3b.
C3b carries out several functions:
- It brings about lysis (bursting) of the target cell by activating subsequent steps in the cascade, leading to the formation of a ringlike structure called the membrane attack complex. This structure, which is composed of complement proteins C5 through C9, inserts itself into the membrane of the invading pathogen and creates a hole through which the cell contents leak out, killing the cell.
- C3b can combine with another protein that converts more C3 protein to C3b.
- C3b can initiate the alternative pathway of complement activation.
- But perhaps the most important result of C3b production is that great numbers of C3b molecules are deposited on the surface of an invading pathogen in a process called opsonization. This makes the microorganism more attractive to phagocytic cells such as macrophages and neutrophils. The attraction occurs because receptors on the surface of phagocytes recognize and bind to the C3b molecule on the surface of the pathogen, stimulating phagocytosis. The microbe is then killed by digestive enzymes present in the phagocytes. If microbes are not immediately killed and are able to reach the bloodstream or the liver, spleen, or bone marrow, they can become coated with antibody and complement there and be ingested by phagocytes.
The small protein fragments that are released during the activation of complement are potent pharmacological agents that help promote an inflammatory response by causing mast cells and basophils to release histamine, which increases the permeability of blood vessels, and by attracting granulocytes and monocytes.
Thus, when a microbe penetrates the body, if antibodies reactive with its surface are already present (or if the microorganism activates complement without the help of antibodies, through the alternative complement pathway), the complete complement sequence may be activated and the microbe killed by damage to its outer membrane. This mechanism is effective only with bacteria that lack protective coats and with certain large viruses, but it is nevertheless important. Persons who lack C3 and thus cannot complete the later steps in the complement sequence are vulnerable to repeated bacterial infections.
Clearly such a biologically important chain of reactions could do more harm than good if its effects were to spread beyond the site of antigen invasion. Fortunately, the active intermediates at each stage in the complement sequence become rapidly inactivated or destroyed by inhibitors if they fail to initiate the next step. With rare exceptions, this confines the activation to the place in the body where it is needed.